Image made using the COMSOL Multiphysics® software and is provided courtesy of COMSOL.
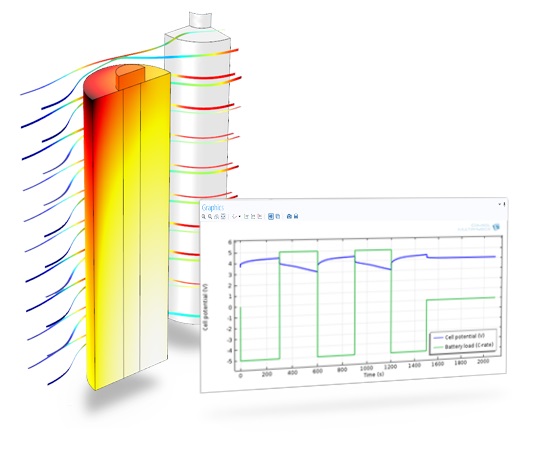
Heat profile in an air-cooled cylindrical lithium-ion battery battery pack. The thermal model is coupled to electrochemical reactions and ion flow, which act as a heat source.
Batteries and fuel cells are being asked to perform in more challenging environments, with greater energy densities or power efficiencies, over longer lifetimes. These requirements are placing more pressure on these industries, and modeling and simulation is fast becoming one of the necessary tools for developing, designing, optimizing, and ensuring quality and safety of batteries and fuel cells during operation. Examples of systems that may be studied include lead-acid batteries, lithium-ion batteries, nickel metal-hydride batteries, solid oxide fuel cells (SOFCs), direct-methanol fuel cells (DMFCs), and proton exchange membrane fuel cells (PEMFCs).
The Batteries & Fuel Cells Module models the underlying electrochemical behavior in the electrodes and electrolytes of batteries and fuel cells. It allows you to investigate their performance for different operating conditions, design configurations, and deterioration due to different aging mechanisms. With this add-on module, you can simulate characteristics such as the transport of charged and neutral species, current conduction, fluid flow, heat transfer, and the nature and driving forces of electrochemical reactions at planar and in porous electrodes. Using this understanding of these characteristics, you can design and optimize the geometries and material choices of your system’s electrodes, separators, membranes, electrolyte, and current collectors and feeders with respect to performance, thermal management, and safety.
Cyclic voltammetry is a common analytical technique for investigating electrochemical systems. In this method, the potential difference between a working electrode and a reference electrode is swept linearly in time from a start potential to a vertex potential, and back again. The current-voltage waveform, called a voltammogram, provides information about the reactivity and mass transport properties of an electrolyte.
The purpose of the app is to demonstrate and simulate the use of cyclic voltammetry. You can vary the bulk concentration of both species, transport properties, kinetic parameters, and the settings of the cyclic voltammeter.
Side reactions and degradation processes may lead to a number of undesirable effects, causing capacity loss in lithium-ion batteries. Typically, aging occurs due to multiple complex phenomena and reactions that occur simultaneously at different places in the battery, and the degradation rate varies between certain stages during a load cycle, depending on potential, local concentration, temperature, and the direction of the current. Different cell materials age differently, and the combination of different materials may result in further accelerated aging due to, for instance, “crosstalk” electrode materials.
This tutorial demonstrates how to model aging in the negative graphite electrode in a lithium-ion battery, where a parasitic solid-electrolyte-interface (SEI) forming reaction results in an irreversible loss of cycleable lithium. The model also includes the effect of increasing potential losses due to the resistance of the growing SEI film on the electrode particles, as well as the effect of a reduced electrolyte volume fraction on the electrolyte charge transport.
This example simulates the heat profile in an air-cooled cylindrical battery in 3d. The battery is placed in a matrix in a battery pack. The thermal model is coupled to a 1d-battery model that is used to generate a heat source in the active battery material.
The model requires the Batteries & Fuel Cells Module and the Heat Transfer Module
The goal with this application is to explain experimental electrochemical impedance spectroscopy (EIS) measurements and to show how you can use a simulation app along with measurements to estimate the properties of lithium-ion batteries. The Lithium-Ion Battery Impedance app takes measurements from an EIS experiment and uses them as inputs. It then simulates these measurements and runs a parameter estimation based on the experimental data.
The control parameters are the exchange current density, the resistivity of the solid electrolyte interface on the particles, the double-layer capacitance of NCA, the double-layer capacitance of the carbon support in the positive electrode, and the diffusivity of the lithium ion in the positive electrode. Fitting is done to the measured impedance of the positive electrode at frequencies ranging from 10 mHz to 1 kHz.
This example shows how to use the Tertiary Current Distribution interface to model the currents and electrolyte mass transport in a thin-film all-solid-state lithium-ion battery.
A separate Transport of Diluted Species interface is coupled to the electrochemical reactions to model the mass transport of lithium in the positive electrode.
Various discharge currents are studied, and the different sources of voltage losses are analysed.
This model simulates a temperature profile in a number of cells and cooling fins in a liquid-cooled battery pack. The model solves in 3D and for an operational point during a load cycle. A full 1D electrochemical model for the lithium battery calculates the average heat source.
The impedance of a lithium-ion battery cell with a negative LTO and positive NCA electrode is modeled for harmonic perturbations between 10 mHz to 1000 Hz. The model incorporates an additional double-layer current at the conductive material in the positive electrode. The impedance of each electrode is also possible to investigate versus a reference electrode located in the middle of the separator.
The model is fitted to experimental data in literature using an optimization interface. Four control variables are used and can be extracted using the optimization procedure. The best fit is achieved using the SNOPT method with a numeric gradient method.
The application takes experimental data from EIS measurements as input, simulates these measurements, and then runs a parameter estimation based on the experimental data.
The control parameters are the exchange current density, the resistivity of the resistive layer on the particles, the double-layer capacitance of NCA, and the double-layer capacitance of the carbon support in the positive electrode. The fitting is done to the measured impedance of the positive electrode at frequencies ranging from 10 mHz to 1 kHz.
During an internal short circuit of a battery, the two electrode materials are internally and electronically interconnected, giving rise to high local current densities. Internal short circuits may occur in a lithium-ion battery due to, for instance, lithium dendrite formation or a compressive shock. A prolonged internal short circuit results in self discharge in combination with a local temperature increase. The latter effect is important because the electrolyte may start to decompose by exothermic reactions if the temperature reaches above a certain threshold, causing thermal runaway with potential health and safety hazards.
This tutorial model investigates the local temperature rise due to the occurrence of a penetrating metallic filament in the separator between the two porous electrode materials. The physics are set up using the Lithium-Ion Battery interface coupled to the Heat Transfer interface. The battery chemistry consists of a graphite negative electrode and an NMC positive electrode with an LiPF6 electrolyte.
In a redox flow battery electrochemical energy is stored as redox couples in the electrolyte, which is stored in tanks outside the electrochemical cell. During operation, electrolyte is pumped through the cell and, due to the electrochemical reactions, the individual concentrations of the active species in the electrolyte are changed.
The state of charge of the flow battery is determined by the electrolyte species concentrations, the total flowing electrolyte volume in the system (tank+pump+hoses+cell), and possibly also by the concentration of solid species on the electrodes. Depending on the cell chemistry the cell can have separated or combined anode and cathode compartments and electrolyte tanks.
This model simulates a soluble lead-acid flow battery during an applied charge-discharge load cycle. The surface chemistry of the positive electrode is modeled by using two different lead oxides and two different positive electrode reactions in the model.
The following example is a 2D tutorial model of a lithium-ion battery. The cell geometry is not based on a real application; it is only meant to demonstrate a 2D model setup.