Image made using the COMSOL Multiphysics® software and is provided courtesy of COMSOL.
Decorative electroplating assuming secondary current distribution with full Butler-Volmer kinetics for both the anode and the cathode. The deposited thickness on the front and the backside of the piece is shown.
Modeling and simulations are cost-effective methods for understanding, optimizing, and controlling electrodeposition processes. A typical simulation yields the current distribution at the surface of the electrodes, and the thickness and composition of the deposited layer. Simulations are used for studying important parameters such as cell geometry, electrolyte composition, electrode reaction kinetics, operating voltages and currents, as well as temperature effects. With information about these parameters, you can optimize the operating conditions of the electrochemical cells and the placement and design of masks, and ensure the quality of your surfaces, while minimizing material and energy losses.
The Electrodeposition Module is suited for a wide variety of applications, including: metal deposition for electronics and electrical parts; corrosion and wear protection; decorative electroplating; electroforming of parts with thin and complex structures; etching; electromachining; electrowinning; and electrorefining. With the Electrodeposition Module, you can consider all of the participating phenomena and simulate them together. More specifically, you may couple the equations that describe current transport and conservation, chemical species transport, charge balances, and electrochemical kinetics. Due to the ability to account for several relevant phenomena, you are able to obtain accurate estimates of the quality, shape, and thickness of the deposit on the surface of the electrodes.
Tools and physics interfaces are available within the Electrodeposition Module for defining the physical characteristics of your process. Predefined formulations allow you to model primary, secondary, and tertiary current distribution effects – often excellent indicators of the surface finish and product quality of your process.
Cyclic voltammetry is a common analytical technique for investigating electrochemical systems. In this method, the potential difference between a working electrode and a reference electrode is swept linearly in time from a start potential to a vertex potential, and back again. The current-voltage waveform, called a voltammogram, provides information about the reactivity and mass transport properties of an electrolyte.
The purpose of the app is to demonstrate and simulate the use of cyclic voltammetry. You can vary the bulk concentration of both species, transport properties, kinetic parameters, and the settings of the cyclic voltammeter.
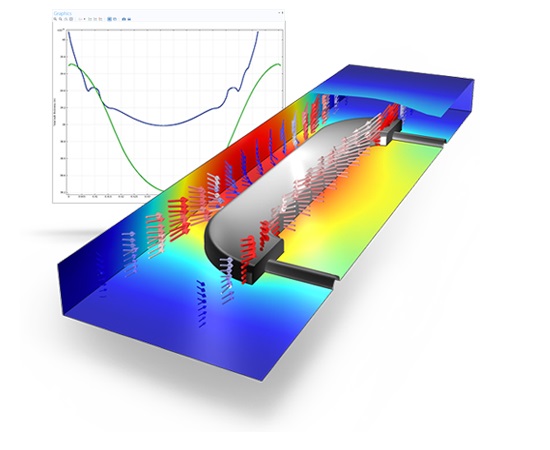
This model demonstrates the use of moving meshes in the application of copper electrodeposition on circuit boards. In these environments, the presence of cavities or ‘trenches’ are apparent.
The model makes use of the Tertiary, Nernst-Planck interface for electrodeposition to keep track of the deformation of the mesh. Furthermore, electrochemical reaction kinetics through use of the Butler-Volmer equation for copper deposition are freely entered at the boundaries.
The model is inherently time-dependent and results clearly show that the mouth of the trench narrows, due to non-uniform deposition of the copper. In addition, the simulation shows a substantial variation in copper ion concentration along the length of the trench. Such effects can be detrimental to the quality of the deposition, create corrosion possibilities, and lead to material waste.
Tutorial model of electroplating. The model uses secondary current distribution with full Butler-Volmer kinetics for both anode and cathode. The thickness of the deposited layer at the cathode is computed as well as the pattern caused by dissolution of the anode surface.
This model demonstrates the impact of convection and diffusion on the transport-limited electrodeposition of a copper microconnector bump (metal post). Microconnector bumps are used in various types of electronic applications for interconnecting components, for instance liquid crystal displays (LCDs) and driver chips.
The location of the bumps on the electrode surface is controlled by the use of a photoresist mask. Control of the current distribution in terms of uniformity and shape is important for ensuring the shape and resulting reliability of the interconnector bumps.
The cell is running at a high overpotential so the deposition rate is governed by the transport rate of the depositing ion in the electrolyte. A result of this operating condition is that the electric potentials in the electrolyte and electrode need not be modeled to determine the current distribution on the bump. The model is based on a paper by Kondo and others.
Rotating cylinder Hull cells are an important experimental tool in electroplating and electrodeposition and are used for the measurement of nonuniform current distribution, mass transport, and throwing power of plating baths. The model reproduces the results for a commercially available cell (RotaHull(R)) as published in paper [1]. In particular, it investigates the primary, secondary, and tertiary current distributions along the electrode as well as the copper diffusion in the diffuse layer around the cathode.
This is a model of the secondary current distribution in a zinc electrowinning cell.
The model investigates the impact on the current distribution when changing the electrode alignment in a parametric study. The geometry is in 2D.
Electrochemical impedance spectroscopy (EIS) is a common technique in electroanalysis. It is used to study the harmonic response of an electrochemical system. A small, sinusoidal variation is applied to the potential at the working electrode, and the resulting current is analyzed in the frequency domain.
The real and imaginary components of the impedance give information about the kinetic and mass transport properties of the cell, as well as the surface properties through the double layer capacitance.
The purpose of the Electrochemical Impedance Spectroscopy analysis app is to understand EIS, Nyquist, and Bode plots. The app lets you vary the bulk concentration, diffusion coefficient, exchange current density, double layer capacitance, and the maximum and minimum frequency.